Week 6 - Model Fitting - Worked Example#
import numpy as np
import matplotlib.pyplot as plt
from scipy.optimize import minimize, curve_fit
Question 1 - The Van’t Hoff Equation#
The Van’t Hoff equation relates the equilibrium constant of a given reaction to the temperature at which that reaction occurs. The linear form can be derived from the two standard expressions for the change in Gibbs free energy:
Given a set of temperatures and equilibrium constants measured from experiment, we should be able to fit the linear form of the Van’t Hoff equation and thus calculate \(\Delta H\) and \(\Delta S\) from the slope and intercept respectively.
We are provided with T and K data for the reaction above.
T / celsius |
K |
|---|---|
9 |
34.3 |
20 |
12 |
25 |
8.79 |
33 |
4.4 |
40 |
2.8 |
52 |
1.4 |
60 |
0.751 |
70 |
0.4 |
The exercise asks us to read data from exercise_1.dat. The file contains temperature and equilibrium constant data in two columns. We can read this using np.loadtxt():
data = np.loadtxt('data/exercise_1.dat', skiprows=1)
temperature = data[:, 0]
eq_con = data[:, 1]
For this worked example, we will enter the data directly:
temperature = np.array([9, 20, 25, 33, 40, 52, 60, 70])
eq_con = np.array([34.3, 12, 8.79, 4.4, 2.8, 1.4, 0.751, 0.4])
The linear form of the Van’t Hoff equation features \(\ln K\) and \(1 / T\), so we need to take the reciprocol of each temperature and the natural logarithm of each K. The temperature is also in celsius, so we need to convert to kelvin. These operations are made trivial by numpy, which allows us to utilise vector arithmetic and operate on each element of an array e.g add 273.15 to each temperature in the temperature array.
temperature_kelvin = temperature + 273.15
inverse_temperature = 1 / temperature_kelvin
ln_eq = np.log(eq_con)
As we would expect, plotting \(1 / T\) against \(\ln K\) gives a linear relationship.
plt.plot(inverse_temperature, ln_eq, 'o')
plt.xlabel('Inverse Temperature / K$^{-1}$')
plt.ylabel('ln K')
plt.show()
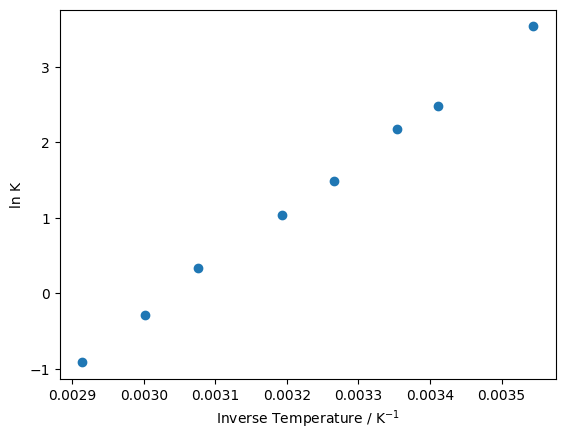
Now we need to “pythonise” the linear form of the Van’t Hoff equation. This is effectively just the equation of a straight line, so we can think of this function as a simple linear model calculating \(y\) (\(\ln K\)) from \(m\) (\(-\Delta H / R\)), \(x\) (\(1 / T\)) and \(c\) (\(\Delta S / R\)).
def linear_model(x, m, c):
"""
Model the equation of a straight line, i.e. calculate y = mx + c.
Args:
x (np.ndarray): x-values
m (float): slope
c (float): intercept
Returns:
np.ndarray: y-values
"""
y = m * x + c
return y
We can test our function with a decent guess as to what \(m\) and \(c\) should be, these are given to us as \(m = 7400\) and \(c = -22.5\). We can calculate our prediction of \(\ln K\) at each value of \(1 / T\) by calling our linear_model function.
m = 7400
c = -22.5
y_fit = linear_model(inverse_temperature, m, c)
plt.plot(inverse_temperature, ln_eq, 'o')
plt.plot(inverse_temperature, y_fit, '-')
plt.xlabel('Inverse Temperature / K$^{-1}$')
plt.ylabel('ln K')
plt.show()
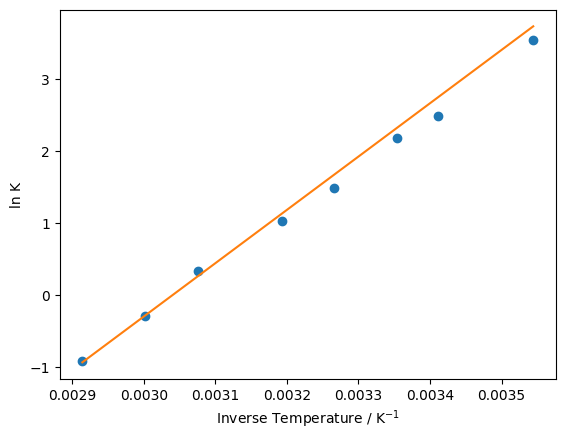
The fit shown above isn’t terrible, but it’s clear that there’s room for improvement. We can define a measure of how good our model of the data is by calculating the sum of squared differences.
Here each \(y_{i}\) is one of our observed values of \(\ln K\) and each \(f(x_{i})\) is the corresponding value that we predict using our linear model. We can pythonise this equation and write a function to calculate \(\chi^{2}\) for us.
def error_function(model_params, x, y):
"""
Calculates the sum of squared differences between the observed y-values and fitted (predicted) y-values.
Args:
model_params (list): m and c
x (np.ndarray): x-values
y (np.ndarray): observed y-values
Returns:
float: sum of squared differences
"""
m, c = model_params
chi_squared = sum((y - linear_model(x, m, c)) ** 2)
return chi_squared
We accept a list of model parameters (model_params) as opposed to \(m\) and \(c\) explicitly due to the fact that we will shortly be using the minimize function from the scipy library. minimize requires the first argument of the input function to be a list containing all of the free parameters that should be adjusted during the minimization procedure. We can use our initial guess for \(m\) and \(c\) from earlier as a starting point for calling minimize.
minimize(error_function, [7400, -22.5], (inverse_temperature, ln_eq))
message: Optimization terminated successfully.
success: True
status: 0
fun: 0.026025756680761968
x: [ 6.924e+03 -2.107e+01]
nit: 4
jac: [ 3.027e-08 -4.424e-06]
hess_inv: [[ 1.540e+06 -4.958e+03]
[-4.958e+03 1.603e+01]]
nfev: 45
njev: 15
We can assign the results output above to a variable and access specifically the optimised parameters as follows:
opt_data = minimize(error_function, [7400, -22.5], (inverse_temperature, ln_eq))
opt_params = opt_data.x
opt_params
array([6924.03005359, -21.06598328])
We can see that minimize has been able to lower the sum of squared differences by adjusting the values of \(m\) and \(c\). Replotting our model against the experimental data, it is clear that the fit is improved relative to what we started with.
m, c = opt_params
y_fit = linear_model(inverse_temperature, m, c)
plt.plot(inverse_temperature, ln_eq, 'o')
plt.plot(inverse_temperature, y_fit, '-')
plt.xlabel('Inverse Temperature / K$^{-1}$')
plt.ylabel('ln K')
plt.show()
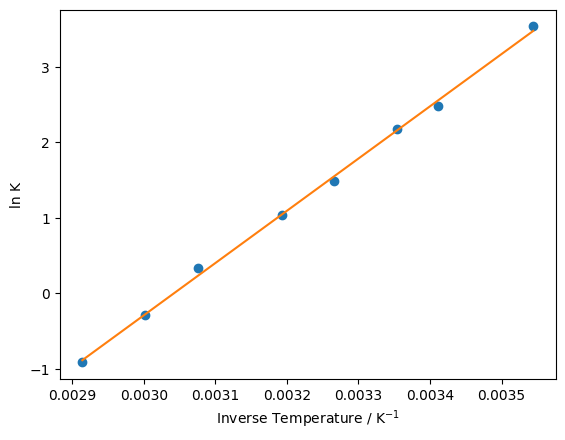
One way to perform linear regression in Python without writing your own model and error functions is to use scipy.stats.linregress(). This function takes the \(x\) and \(y\) data directly and returns the slope and intercept (along with some additional statistics).
from scipy.stats import linregress
result = linregress(inverse_temperature, ln_eq)
print(f"Slope (m): {result.slope}")
print(f"Intercept (c): {result.intercept}")
Slope (m): 6923.875242734125
Intercept (c): -21.065484494329493
Reassuringly, these values are essentially identical to those we obtained using minimize(). The small differences are due to numerical precision in the optimisation algorithm.
Question 1.5 - Bonus Round#
Having fit the Van’t Hoff equation to our experimental data - we can easily extract \(\Delta H\) and \(\Delta S\).
The enthalpy change is contained within the gradient.
Whilst the entropy change is related to the intercept.
Let’s calculate these quantities from our optimized values of \(m\) and \(c\).
from scipy.constants import R
m, c = opt_params
delta_H = -R * m
delta_S = R * c
print(f'Delta H = {delta_H} J / mol or equally {delta_H / 1000} kJ / mol')
print(f'Delta S = {delta_S} J / molK')
Delta H = -57569.58904756049 J / mol or equally -57.56958904756049 kJ / mol
Delta S = -175.15233048994133 J / molK
Question 2 - A First-Order Rate Equation#
The procedure we followed above is not limited to linear systems, we can just as easily fit a non-linear model by minimizing the sum of squared differences between that model and the experimental data. In this question, we are concerned with fitting a first-order rate equation to a series of time and concentration data.
We are provided with the relevant data for the decomposition of hydrogen peroxide in the presence of excess Ce\(^{\text{III}}\).
Time / s |
[H\(_{2}\)O\(_{2}\)] / moldm\(^{-3}\) |
|---|---|
2 |
6.23 |
4 |
4.84 |
6 |
3.76 |
8 |
3.20 |
10 |
2.60 |
12 |
2.16 |
14 |
1.85 |
16 |
1.49 |
18 |
1.27 |
20 |
1.01 |
As with Exercise 1, we would normally read the data from the file exercise_2.dat:
data = np.loadtxt('data/exercise_2.dat', skiprows=1)
time = data[:, 0]
concs = data[:, 1]
For this worked example, we will enter the data directly:
time = np.array([2, 4, 6, 8, 10, 12, 14, 16, 18, 20])
concs = np.array([6.23, 4.84, 3.76, 3.20, 2.60, 2.16, 1.85, 1.49, 1.27, 1.01])
We can now plot time against [H\(_{2}\)O\(_{2}\)].
plt.plot(time, concs, 'o')
plt.xlabel('Time / s')
plt.ylabel('[H$_{2}$O$_{2}$] / mol dm$^{-3}$')
plt.show()
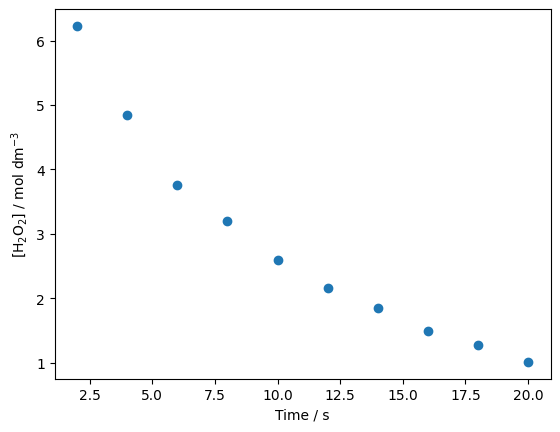
Sure enough, it doesn’t look linear this time - but we press on. Time to pythonise the first-order rate equation.
def first_order(time, init_conc, k):
"""
Model a first-order rate equation to predict [H2O2] from t.
Args:
time (np.ndarray): time values
init_conc (float): [H2O2]0 i.e. the initial concentration of hydrogen peroxide
k (float): rate constant
Returns:
np.ndarray: [H2O2] for each input t
"""
concs = init_conc * np.exp(-k * time)
return concs
We can also define an error_function to calculate the sum of squared differences, this time around we call our first_order function in place of the linear_model, but the concept is very much the same.
def error_function(model_params, time, concs):
"""
Calculate a sum of squared differences between the observed concentration of H2O2
at time t and the corresponding values predicted via a first-order rate equation.
Args:
model_params (list): initial concentration of H2O2 and the rate constant
time (np.ndarray): time data
concs (np.ndarray): observed concentration data
Returns:
float: sum of squared differences
"""
init_conc, k = model_params
chi_squared = sum((concs - first_order(time, init_conc, k)) ** 2)
return chi_squared
Once again, we minimize our error function with respect to the free parameters of our model - in this case init_conc ([H\(_{2}\)O\(_{2}\)]\(_{0}\)) and k (the rate constant).
minimize(error_function, [7, 0.1], (time, concs))
message: Optimization terminated successfully.
success: True
status: 0
fun: 0.13373044840256892
x: [ 7.422e+00 1.031e-01]
nit: 6
jac: [ 9.313e-09 3.632e-07]
hess_inv: [[ 7.694e-01 1.231e-02]
[ 1.231e-02 2.978e-04]]
nfev: 33
njev: 11
It looks like our minimization worked just fine. Now we can use the optimized parameters to plot our model alongside the experimental data.
opt_data = minimize(error_function, [7, 0.1], (time, concs))
opt_params = opt_data.x
init_conc, k = opt_params
fitted_concs = first_order(time, init_conc, k)
plt.plot(time, concs, 'o')
plt.plot(time, fitted_concs, '-')
plt.xlabel('Time / s')
plt.ylabel('[H$_{2}$O$_{2}$] / mol dm$^{-3}$')
plt.show()
print(f'[H2O2]0 = {init_conc} mol / dm^3')
print(f'k = {k} s^-1')
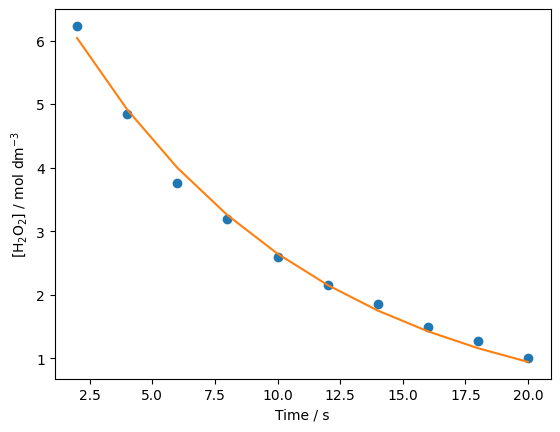
[H2O2]0 = 7.42164402082281 mol / dm^3
k = 0.10308255365613304 s^-1
We can also perform non-linear least-squares fitting without writing an explicit error function by using scipy.optimize.curve_fit(). Here, we pass our starting guesses via the optional p0 parameter to help the optimiser converge efficiently.
from scipy.optimize import curve_fit
popt, pcov = curve_fit(first_order, time, concs, p0=[7, 0.1])
print(f"[A]_0 = {popt[0]}")
print(f"k = {popt[1]}")
[A]_0 = 7.421644124098848
k = 0.10308255602605224
The first array returned by curve_fit contains the optimised parameters—reassuringly, these match the values we obtained using minimize(). The second array is the covariance matrix, which provides information about the uncertainties in the fitted parameters.
