Exponentials#
We may use the term “exponential growth” as a way to describe the rate of a reaction as the temperature increases. In using this phrase, we are describing a very fast rate of increase. We can represent this idea mathematically with one of the simplest exponential equations:
where, \(a\) is a constant, \(x\) is the controlled variable (in the above case, temperature), and \(y\) is the observed variable (in the above case, rate of reaction).
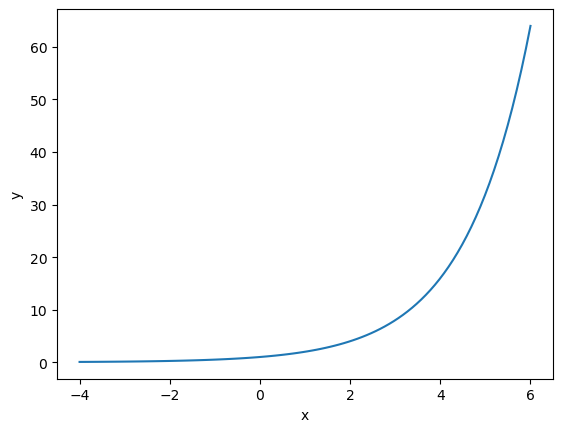
Above, we have the graph of \(y=2^x\) and we can see the graph increases at a very fast rate, which is what we would expect with exponential growh. Other mathematical things to note are:
The graph passes through the point (0, 1), as any variable raised to the power of 0 is equal to 1.
The values of \(y\) are small and positive when \(x\) is negative.
The values of \(y\) are large and positive when \(x\) is postive.
The Exponential Function#
So far, we have used the word exponential to describe equations in the form \(y=a^x\). However, the word is usually reserved to describe a special type of function. It is quite likely we will have see the symbol π before, and know it represented the irrational number 3.14159265…. In this section, we will be working with the number:
Like π, e is another number that goes on forever! when we refer to the exponential function, we mean \(y=e^x\). This can be seen as a special case of the previous section, when the constant is \(a=e\), in the equation \(y=a^x\). When we refer to \(e^x\), we say “e to the x”.
There are two ways to access the value e in Python.
import numpy as np
np.e
2.718281828459045
np.exp(1)
2.718281828459045
Arrhenius Equation
The Arrhenius equation below describes the exponential relationship between the rate of a reaction \(k\) and the temperature \(T\).
where \(R\), \(E_a\) and \(A\) are all constants. Support for a reaction that the activation energy is \(E_a=52.0\;\textrm{kJ mol}^{-1}\), the gas constant \(R = 8.314\;\textrm{JK}^{-1}\textrm{mol}^{-1}\) and \(A = 1.00\). What is the rate of the reaction \(k\), when the temperature \(T=241\;\textrm{K}\)?
Solution: We substitute our given values from the question into the Arrhenius equation.
Python is great for doing computations such as these.
from scipy.constants import R
E_a = 52e3
T = 241
A = 1
k = A * np.exp(-E_a / (R * T))
k
5.3663046790901465e-12
Exponential Graphs#
Exponential graphs follow two general shapes, called growth and decay, which are shown below.
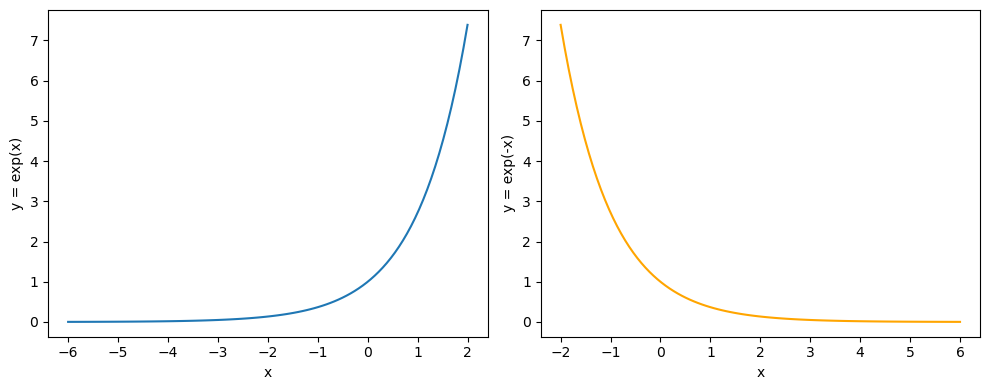
On the left exponential growth, which is represented by the equation \(y=e^x\), note that the graph gets steeper from left to right.
On the right exponential growth, which is represented by the equation \(y=e^{-x}\), note that the graph gets shallowed from left to right.
Alegbraic Rules for Exponentials#
We use very similar rules as the ones we had for powers. The only difference is that the base is now a constant and the power is a variable.
\(a^x \times a^y = a^{x+y}\)
\(\frac{a^x}{a^y} = a^{x-y}\)
\((a^x)^y = a^{x\times y}\)
Example
Simplify the following equations:
\(y = 3^x \times 3^{2x}\)
\(y = \frac{2^x}{4}\)
\(y = \left(e^{-x+1}\right)^x\)
Solution:
Since the bases ar ehte same, we use the first rule.
\[ y = 3^x \times 3^{2x} = 3^{x+2x} = 3^{3x} \]The first thing we notice is that \(4\) can be written as \(2^2\) so:
\[ y = \frac{2^x}{2^2} \]Now that we have powers to the same base, we can apply the rules. Using the second rule, we have:
\[ y = 2^{x-2} \]Using the third rule, we get that:
\[ y = \left(e^{-x+1}\right)^x = e^{-x^2+x} \]
The sympy package is aware of these rules.
from sympy import symbols
x = symbols('x')
3 ** x * 3 ** (2 * x)
from sympy import simplify
simplify(2 ** x / (2 ** 2))
Unfortunately, the final example does not work with sympy.
